
This cookie is set by GDPR Cookie Consent plugin. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. Attacking otherwise-inert materials such as glass, and forming compounds with the usually inert noble gases. What is the most active chemical element known as halogen?įluorine is the most active of all chemical halogen elements. One of the most reactive halogen elements is the fluorine. They react to metals and to nearly all non-metals except oxygen, neon, nitrogen and helium. Halogen atoms have a high effective nuclear charge that makes them highly electronegative which in turn causes the halogens to become highly reactive elements. Why are halogens the most reactive of the nonmetal elements?

These elements reacts with metals to produce salts. The choices given belongs to the group of halogens which means salt-producing. Fluorine is the most reactive among the choices.
Halogen reactivity free#
The free element is widely used as a water-purification agent, and it is employed in a number of chemical processes. Chlorine is the best known of the halogen elements. Which halogen has the greatest reactivity?įluorine is the most reactive of the halogens and, in fact, of all elements, and it has certain other properties that set it apart from the other halogens. This is due to greater stability of 3∘-carbocation formed in the first step. Most reactive halide towards S_(N^(1)) reactions is Tertiary butyl chloride is most reactive in SN1 reaction. Which halogen is more reactive in SN1 reaction? Is Cl or Br more reactive?Īlthough the bromine nucleus is more positively charged than the chlorine nucleus, the increase in the radius and the extra shielding in the bromine atom outweigh this factor, which means that an electron is more easily attracted into the outer shell of a chlorine atom than that of a bromine atom, so chlorine is more … Halogens are more reactive than hydrogen because, in case of halogens they have 7 valence electrons in their valence shell so they acquire 1 more electron to complete their octet attaining noble / inert gas configuration. Which is more reactive halogen or hydrogen?
Its chemical activity can be attributed to its extreme ability to attract electrons (it is the most electronegative element) and to the small size of its atoms. The reaction is faster.įluorine (F), most reactive chemical element and the lightest member of the halogen elements, or Group 17 (Group VIIa) of the periodic table. Has to be warmed and the iron wool heated. Reacts with heated iron wool very quickly.

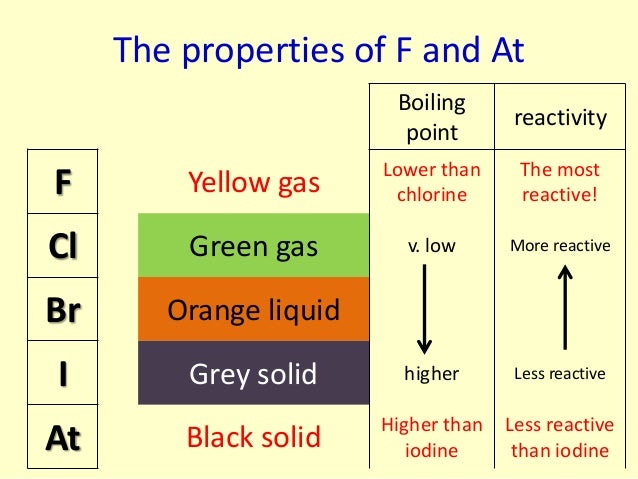
Which is the third most reactive halogen?įluorine is the most reactive element of all in Group 7….Reactivity of halogens. The order of reactivity is chlorine > bromine > iodine. Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Fluorine is one of the most reactive elements. Halogens can gain an electron by reacting with atoms of other elements. This reactivity is due to high electronegativity and high effective nuclear charge.

Halogens are highly reactive, and they can be harmful or lethal to biological organisms in sufficient quantities. What is the order of reactivity of halogens?


 0 kommentar(er)
0 kommentar(er)
